Nutraceuticals have been increasingly popular in recent years, with individuals using them for a range of therapeutic purposes. The rise of the Nutraceuticals sector is being driven not only by industrial innovation and the creation of new products to fulfil the demands of health-conscious consumers, but also by health claims including a wide variety of wellness components. Nutraceutical products have been demonstrated to enhance health outcomes by lowering the risk of cancer, heart disease, and other linked conditions including cataracts, menopausal symptoms, sleeplessness, poor memory and attention, and gastrointestinal issues. Furthermore, cardiovascular agents, anti-diabetic agents, anti-cancer agents, immune boosters, anti-obese agents, substances that manage chronic inflammatory disorders, and formulations provide numerous therapeutic benefits as well as physiological benefits or protection against various diseases.


Nutraceuticals are a potentially growing sector that combines medical treatment and nutrition to give holistic medical care. These can be used as dietary supplements, to prevent diseases like CVD, to support and cure many types of cancer, and to give other healthcare benefits. As a result, the nutraceutical sector now recognises and anticipates the potential success of nutrients that impact individuals healthcare. Conducting clinical research is critical in a highly competitive market to give scientific evidence on projected benefits. We at ICBio will help to prove the safety and efficacy of Nutraceuticals goods, as well as label scientifically proved items, therefore delivering a good product to mankind. Scientific research confirms that the improved safety and efficacy of newly developed nutraceutical products, and their applications in nutrition development and healthcare. Scientific study confirms that newly produced nutraceutical products have improved in terms of safety and efficacy, as well as their uses in nutrition development and healthcare.
In 2008, ICBio launched its clinical activities by conducting its first herbal clinical study. Since then, ICBio has had a committed and skilled staff undertaking nutraceutical and herbal clinical investigations. Since then, multiple successful executions have occurred for items relating to sports nutrition, nutritional supplements, herbal anti-diabetics, herbal extracts for gastro-esophageal reflux illness, energy booster supplements, slimming supplements, sexual dysfunction, probiotics, and so on. Our experienced workforce combines expertise and ability, which are trademarks of optimising long-term collaborations. ICBio CRO is expanding its services to include the design and execution of clinical studies for herbal and nutraceutical products. We understand the complicated difficulties that arise during the life cycle of herbal and nutraceutical products, and we provide new solutions by merging our many services.


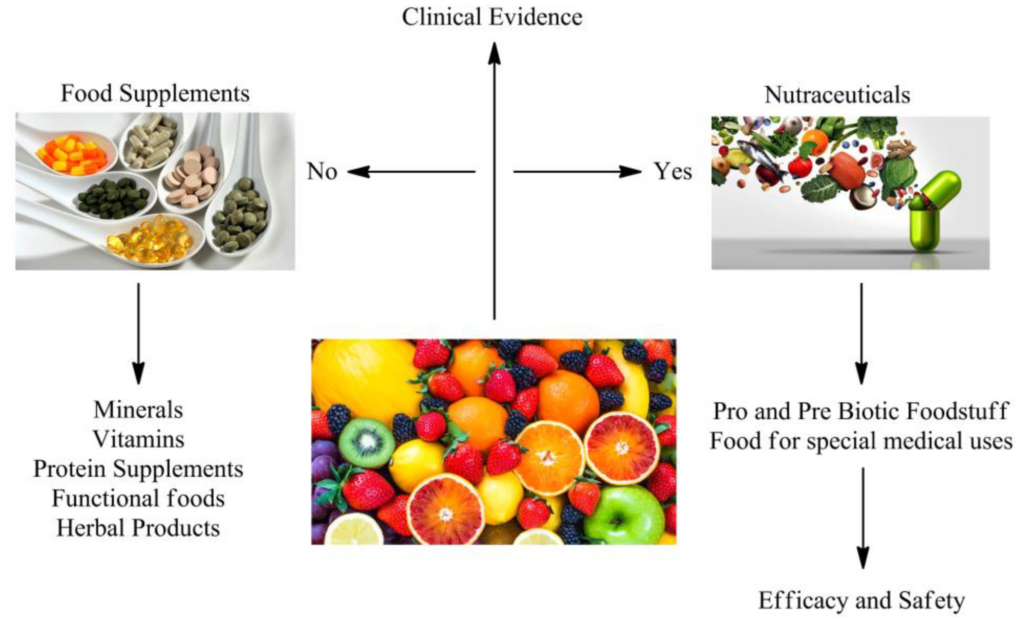
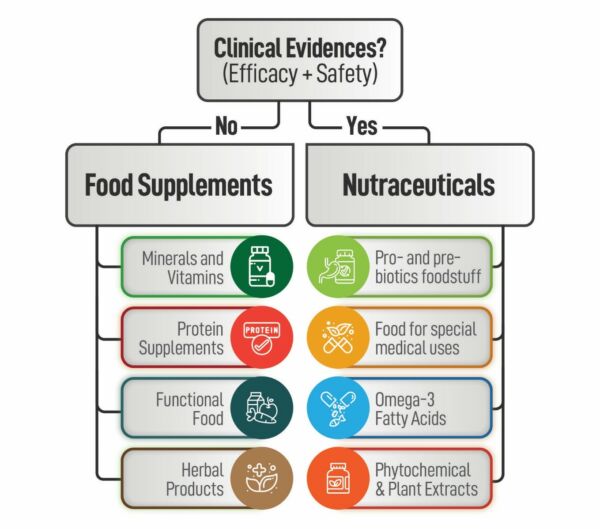
Herbal and nutraceutical products have grown in popularity in recent years, and companies are looking for the most efficient ways to develop products. As an innovative Contract Research Organisation, we are always looking for new opportunities to provide our clients with the most up-to-date services in a variety of areas. The absence of scientific and clinical evidence, as well as a greater knowledge of the efficacy and safety of herbal products, is a fundamental impediment to the incorporation of herbal medicines into modern medical practises.
It is not true that “if a poly herbal product is prepared according to the traditional system of medicine, it is bound to be safe and effective.” If you want a herbal product to stand out from the crowd, it must make a health benefit claim. Such health claims can be supported by well- designed Double Blind Randomised Clinical Trials. Because of its contemporary facilities, large patient population, and superior data administration abilities, India has quickly become a popular location for Clinical Research.
We at IC BIO CRO have a procedure in place to fulfil the unique needs of our clients in the field of clinical registration of novel pharmaceutical products from phase to phase IV, including bioequivalence, bioavailability, pharmacokinetic/pharmacodynamics studies, Statistical Analysis, and Data Management in accordance with ICH and GCP standards. The firm provides local and international pharmaceutical and bio-pharmaceutical enterprises with competitive and high-quality clinical trial services.
Recent adverse events have clearly shown that all herbal products are not free from side effects and toxicity. Products that have been confirmed to have bio efficacy can then be taken up for safety studies to determine possible side effects, adverse reactions and the maximum tolerable dosages. We at ICBIO undertake systematic animal studies of herbal extracts and formulations by utilizing appropriate models. Toxicological Studies at our Laboratory are performed as per OECD, ICH, MHW, TGA, AYUSH, Schedule Y, etc.
Few of the types of toxicological/animal studies regularly performed are as follows.
Submit your details and we’ll be in touch shortly. You can also email us if you would prefer.
Copyrights © 2012 – 2023 ICBIO Corporation